HandsOn 13 - Growing a Pattern in the Laboratory
I. Introduction
I. Introduction
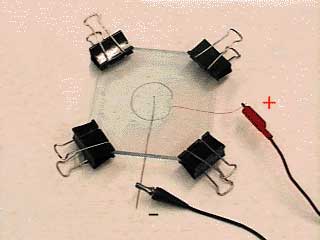
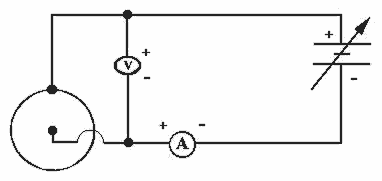
In this experiment, supplied in the accompanying laboratory kit, you have the opportunity to grow a physical object and measure its fractal dimension by two independent methods. The electrochemical deposition experiment is abbreviated ECD. The ECD experiment is carried out in an ECD cell consisting of two parallel plastic plates (Figure 4.1). Between these plates a circular positive terminal surrounds a central negative terminal. The space between the plates is the thickness of the positive terminal wire, about 1/2 mm or 500 micrometers. Between the plates, and between the electrodes, is a salt solution. Most likely you will be using a solution of copper sulfate (Cu2SO4) or zinc sulfate (Zn2SO4). This apparatus is an electrolytic cell, one that requires a current input (as opposed to an electrochemical cell such as a battery-a galvanic cell-which spontaneously produces a current). The circuit is shown in Figure 4.2.
|
 |
 |
|
|
|
If you are working in a classroom setting, you will need at least one partner,
and preferably two partners, for this experiment. In addition to the equipment
provided in the kit, you will need a power source with variable voltage
(e.g., between 1 and 20 Volts) and current (between 10 and 250 milliamps).
Previous: 4.1 - Electrodeposition