HandsOn 17 - Modeling a Rough Surface
As part of your data analysis you will measure the fractal dimension of the surface, or perimeter, of the bacterial colony. The question is whether the colony interface may reflect social interactions, or whether the spread of the colony is effectively random.
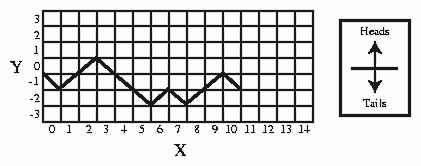
A random surface can be generated by the following random walk exercise. On a piece of graph paper, draw a horizontal line across the middle. This is your y = 0 line. Make a mark at the leftmost point of the axis. This is your origin (x = 0, y = 0). Move your marker over one unit to x = 1. Flip a coin. If heads results, move up to y = +1; if tails, move down to y = -1. Move your marker over to x = 2. Flip the coin. If it is heads, move y up one; if tails, move y down one. Repeat this process moving your marker over one unit on the x-axis each time (the x-axis represents time), and up or down one unit in the y-direction, depending on the result of the coin flip. For example, if you flip THHTTTHTHHT then the connected points make a graph like Figure .
 |
The result is a random surface. Connect your points with dark magic marker, scan it into the computer with a contrast setting so that the grid vanishes, and find the dimension using the Fractal Dimension program.
|
|
A common model of molecules in gases and liquids shows particles
constantly in motion, dancing around due to thermal disturbances.
In a uniform gas or liquid nothing changes, on average, because
of this motion; on average, equal numbers of molecules move in and
out of a given volume. Since the motion is random, like that of
a random walker, there is no "current,'' no net directed
flow of molecules in a given direction. This is a picture of a medium
that is "at equilibrium.''
Now extend this mental model to the case in which a concentration difference exists. For example, suppose we have a barrier between two containers of water. The container on the left has salt dissolved in the water; the one on the right is pure distilled water. We now raise this barrier carefully, so as not to cause fluid motion.
|
When a concentration difference occurs between regions of the same
medium, that medium is no longer in equilibrium. The result is a
net diffusion of particles from the high-concentration region to
the low-concentration region.
|
In HandsOn 18 you will be growing bacteria under
various nutritional conditions. You will prepare agar plates to grow the bacteria
and you will create streak plates to produce individual colonies of genetically
identical bacteria.
Previous: 5.1 - Bacterial Colonies