HandsOn 22 - The Diffusing Checkers Model
The ammonia molecules and the hydrogen chloride molecules move in the same way that air molecules move. All molecules follow jagged paths because they collide with one another. We do not have the computing power or the numerical accuracy to follow each particle in order to predict every collision and its consequences. Instead, we model the diffusion process by assuming that the motion of each particle is a random walk (Units 2 and 3). Is this a good model? Lets find out.
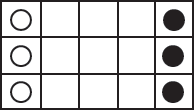
Use the corner of a checkerboard or draw a grid to set up the array of checkers shown in Figure . In this figure, black checkers are shown black and red checkers are shown as unfilled circles. One color of checker, say the red, represents a hydrogen chloride (HCl) molecule. The other color checker, the black, represents an ammonia molecule (NH3). They diffuse into the center from both sides.
 |
Now put your finger on each checker in turn and flip a coin. If the coin comes up a head, move that checker to the right. If the coin is a tail, move the checker to the left. Exceptions: If a head occurs for any checker in the right-hand column, the checker cannot move right, so that checker takes no step and "loses its turn.'' Go on to the next checker. Similarly, if a tail occurs for any checker in the left column, it cannot move left, so does not move at all during that turn.
Whenever a black checker and a red checker move into the same square, they have a chemical reaction, bond together, and stop moving. For example, in Figure one square in the bottom row contains both a black checker and red checker.
 |
In this case the two join together and stop moving. The joined checkers represents a molecule of the solid ammonium chloride (NH4Cl) dust that results when a red-checker HCl molecule and a black-checker NH3 molecule combine chemically.
The single checkers that remain on the grid continue to move by the same coin-flipping rules until every checker is bonded with another (by moving into the same square).
Record in which columns the "bonded molecules'' are distributed. Then repeat the exercise from the beginning and record the distribution of the bonded molecules in the second set. Are the two sets of results similar or different? If several groups have done the exercise, sum and tabulate the results to see if a pattern emerges in the positions of the bonded molecules.
Previous: 6.1 - The Diffusion Chamber